At this station, your team will measure the density of water, compute the
salinity (saltiness) of water, and learn what these measurements tell about the
water at your aquatic site. To do this, you will build and use a hydrometer, a
device that measures the density (or weight of molecules) in different liquids
as compared to water. From these measurements, you'll be able to compute
the salinity of the water. Most living creatures can tolerate only a narrow
range of salinity. If the salinity in a body of water is too high or too low, many plants and animals will die.
Materials
For the Pre-Field station:
- Large plastic drinking straw
- Modeling clay or plasticine, small piece
- Cup of sand
- Four 1-liter beakers or jars. one each containing water, milk, alcohol, and ice water
- Black waterproof marker
- Table salt, 3/4 liter (3 cups)
- Glass stirring rod or plastic coffee stirrer
- Copy of Master 4e, "Density and Salinity Conversion Chart," one for each research team
- Hot water
- Distilled water, 750 ml
For the field station:
- 1-liter beaker or jar, empty
- Hydrometer
- Your team's copy of Master 4a, "Baseline Study and Possible Human Impact
Assessment Form"
- One copy of Master 4e, "Density and Salinity Conversion Chart," for each
research team
Methods
Pre-Field Experiment:
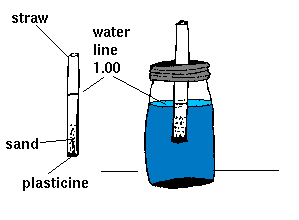
First you need a hydrometer. If it has already been built by another group,
review steps 1 through 5 and begin at step 6.
- Plug one end of the straw with a small piece of modeling clay or plasticine.
- Fill the straw one-quarter full with dry sand.
- Fill the empty beaker or jar three-quarters full with distilled water (750 ml).
Put the straw in the beaker, with the modeling clay at the bottom. Be
careful not to let water get into the open end of the straw.
- Look at how the straw is floating in the water. Is half of the straw above the
waterline and half below the waterline? If not, add or subtract sand from
the straw until it floats with exactly half of the straw above the water and
half below the water.
- Using the waterproof marker, make a horizontal line on the straw at the
waterline. Label the line "1.00." This is your hydrometer.
- Try floating the hydrometer in the jars containing the other liquids (milk,
alcohol, and ice water). The chart below indicates the density of each of
these liquids. Mark the waterline for each substance and write the
appropriate density number. Key this to different colors.
- To see how salinity affects water density, empty the beaker from step 3 and
refill it three-quarters full (750 ml) with room-temperature water. Slowly
add salt to the water, and occasionally stir the solution until the water
becomes saturated. You will know when the water has become saturated,
because the salt you are adding will begin to accumulate at the bottom of
the beaker and no longer dissolve.
- Float the hydrometer in the saltwater. Does the 1.0 mark float higher or
lower than it did in step 3? Using the waterproof marker, mark the new
waterline on the straw.
- To see how temperature affects water density, empty the beaker and refill it
three-quarters full with hot water. Put your hydrometer in the beaker and
note where the waterline is. How does it compare to the waterlines for ice
water and room-temperature water? How does it compare to the waterline
for milk? What does this tell you about the effect of temperature on water
density?
- You will take you hydrometer to the aquatic site to help you measure the
salinity of water there. First, you will record the density of the water. Then
you will use Master 4e, "Density and Salinity Conversion Chart" to convert
that reading into a measure of salinity. Practice using Master 4e now by
converting the density measurements for distilled water, ice water, alcohol,
and milk to salinity measurements.
Field Experiment
To measure the salinity of the water at your aquatic site you will need a 1-liter beaker or jar and your hydrometer.
- Fill the beaker or jar three-quarters full (750 ml) with water from the
aquatic site.
- Place the hydrometer in the water. Which of the marks is the surface of the
water closest to? In other words, is the density greater than or less than 1.0?
- Using Master 4e, "Density and Salinity Conversion Chart," convert your
density reading into a salinity measurement. How does the salinity at your
site compare to the salinity measurements you calculated for various
substances in the classroom?
Conclusions
After you have conducted both the Pre-Field and Field Experiments, discuss
the following questions within your research team and record your answers
in your JASON Journal.
- Does a hydrometer float higher in dense water than in less dense water?
What does higher density indicate about the water?
- Describe the relationships among the following three abiotic characteristics:
density, salinity, and temperature. (Hint: If one characteristic, such as
temperature, increases, what happens to the others?)
- What new questions do you have?
- Look for more answers to your questions in the JASON Online Systems,
the library, and science textbooks.

Return to the Aquatic Field Investigation
JASON VII Home Page

JASON Project Homepage ||
Teachers' Guide ||
Students' Corner ||
Search
Gene Carl Feldman
(gene@seawifs.gsfc.nasa.gov)
(301) 286-9428
Todd Carlo Viola, JASON Foundation for Education (todd@jason.org)
Revised: 3 Nov 1995


![]()
![]()